This carbon here would form five bonds so would violate octet rule. So this one is the government structure of the given company.
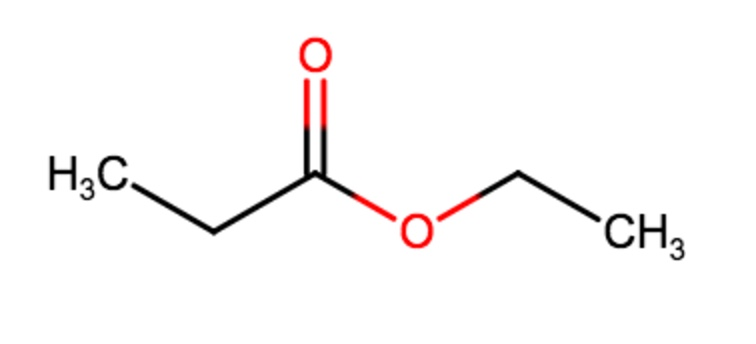
Solved Helppp Draw The Resonance Structure Of The Chegg Com
Write the CSS and draw the resonance hybrid of each of the following.

. How to Draw Resonance Structures Lewis Structures Example 1. Draw the resonance structure of the following substance. Draw the resonance structure of the following substance.
So then that means this double bond that the carbon has well need to get pushed up. View solution Find the substance which can form a resonance structure. The resonance hybrid of ozone has a 1 charge associated with the oxygen at the centre and a partial charge of -½ associated with the other oxygen atoms.
Draw the resonance structure of the following substance. Interactive 3D display mode HC CH3 Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars including charges where needed. But to identify each resonance structures it is good to show arrows.
There are many types of the carbocation is formed in different chemical reactions. Draw one resonance structure of the following substance different from the given one Interactive 3D display mode oll НАС CH3 Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars including charges where needed. H C 1 N HC CH3 o.
View solution In the following the least stable resonance structure is. In drawing resonance structures for a molecule we are only allowed to move electrons. Drawing the Lewis Structure of Ozone.
All of the resonance structures must be proper Lewis structures. Interactive 3D display mode Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars including charges where needed. This molecule has two extra electrons.
Write resonance structure of the given compound. Oc 0 h 120 ex cont с n o hc сн. Carbon atom 4 Oxygen atoms 36 18 For -2 charge 2 Consider the -2 charge at the last step ie.
Draw the resonating structure of c6h5no2 and c6h5oh. The single bond is active by default. Show transcribed image text.
Yeah thats the sp minus. Draw the resonance structure of the following substance It is simpler than uncomplicated to create superb nail artwork for short nailsAll you need to do will be to introduce some glitter in. Combine the resonance structures by adding dotted bonds where other resonance bonds can be formed.
Draw the resonance structure of the following substance. The following geometries may be used. LIMITED TIME OFFER.
43 Years JEE ADVANCED 1978-2020 JEE MAIN. CO32-ion Step 1 Calculate the total number of valence electrons from each atom. Draw the resonance structure of the following substance.
Since the molecular formula is O 3 we know there are 18 valence electrons oxygen has six valence electrons as 6 x 3 18. No that would give us a resident structure of must destroy all the skeleton again. And the only place you can get really move is to right here.
In resonance structures it does not require to show transformation of electrons by arrows. What molecules dont compare then contribution will be possible. Add only the lone pairs found on ALL resonance structures.
The two resonance structures of the ozone molecule are illustrated below. Oc 0 H 120 ex cont с N O HC сн. This carbon here would form five bonds so would violate octet rule.
Draw the resonance structure of the following substance. After placing all the electrons we will have a double bond and a single bond. To draw all resonance structures take the lewis structure we drawn by using VESPR rule.
Draw the resonance structure of the following substance. Up to 256 cash back Get the detailed answer. To find the resonance structure of ozone we will draw the lewis structure of ozone.
And the only place you can get really move is to right here. I CH2 CH - Cl. This carbon here would form five bonds so would violate octet rule.
Click hereto get an answer to your question Draw the resonance structures of the following compounds. Drawing correct resonance structures. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
The single bond is active by default. View solution View. Ii CH2 CH - CH CH2 iii CH2 CH - H C O Solve Study Textbooks Guides.
So we still have are two lone pairs right there. The bottom is the finished resonance hybrid for CO32-. The single bond is active by default.
Draw a resonance structure of the following. We can draw three resonance structures for SO 2 molecule. Draw the resonance structure of the following substance.
No that would give us a resident structure of must destroy all the skeleton again. Draw the Lewis Structure Resonance. The positions of all nuclei must remain the same.
Draw a resonance structure of the following. I CH2 CH - Cl. View solution Resonance structures differ only in the arrangement of _____.
For example we should not write a structure in which carbon has five bonds. Interactive 3d display mode draw the molecule on the canvas by choosing buttons from the tools advanced template toolbars including charges where needed. So then that means this double bond that the carbon has well need to get pushed up.
Resonance Structures of Carbonate CO 3 2 Ion. In following examples arrows are used to show electrons transformation. Ii CH2 CH - CH CH2 iii CH2 CH - H C O.
So we still have are two lone pairs right there. Write the CSS and draw the resonance hybrid of each of the following. Draw the resonance structure of the following substance.
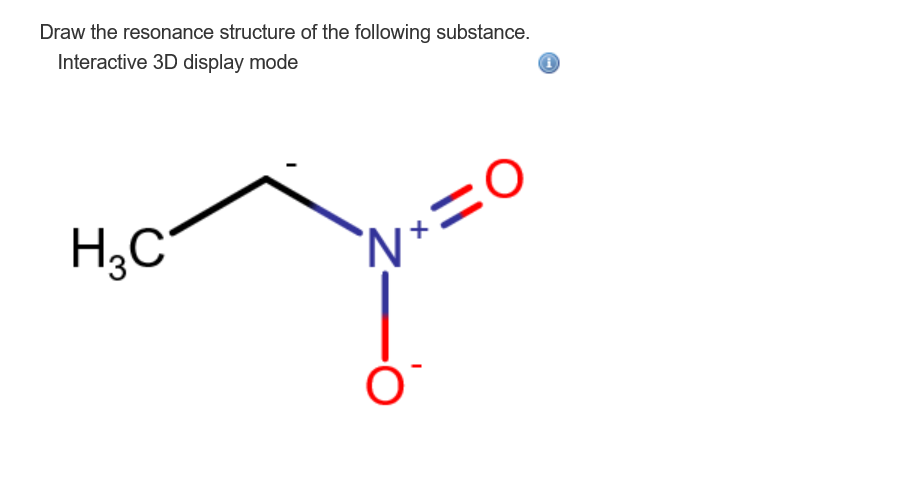
Solved Draw The Resonance Structure Of The Following Chegg Com

Resonance Structures Basic Introduction How To Draw The Resonance Hybrid Chemistry Youtube
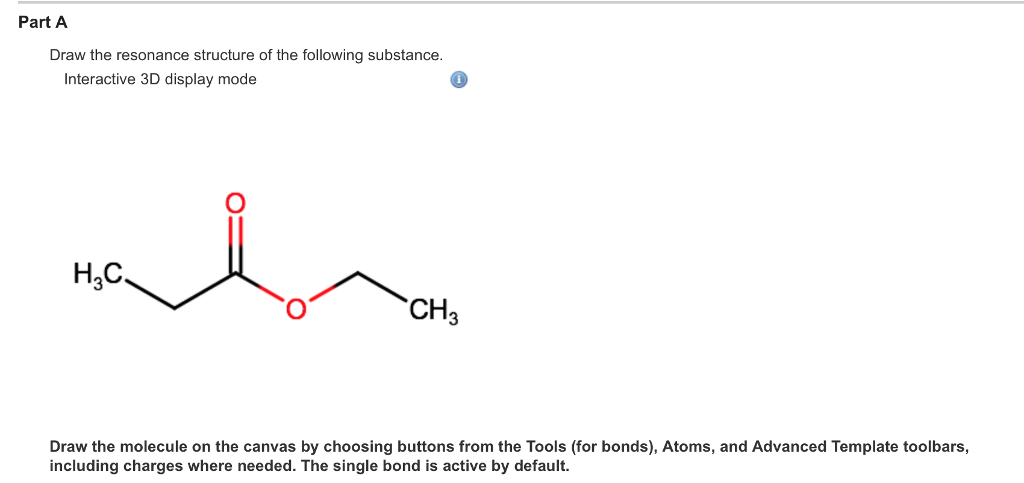
Solved Draw The Resonance Structure Of The Following Chegg Com
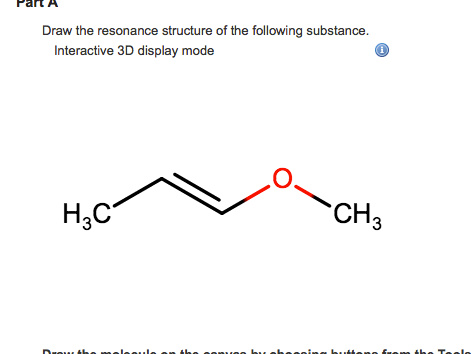
Solved Draw The Resonance Structure Of The Following Chegg Com
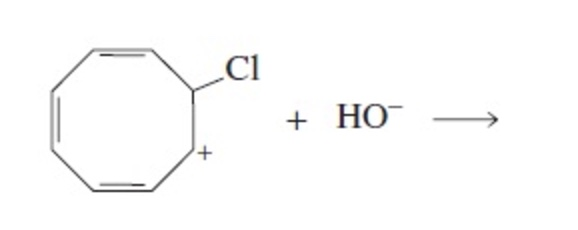
Solved Helppp Draw The Resonance Structure Of The Chegg Com
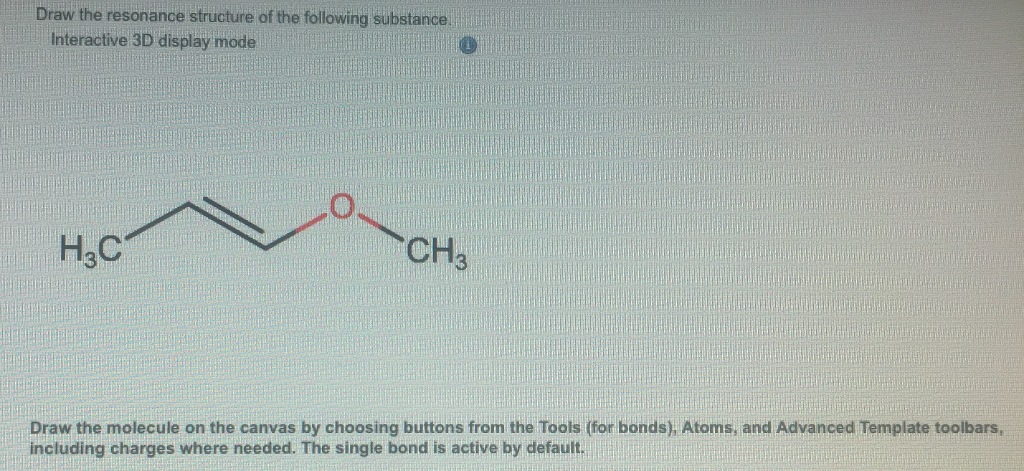
Solved Draw The Resonance Structure Of The Following Chegg Com
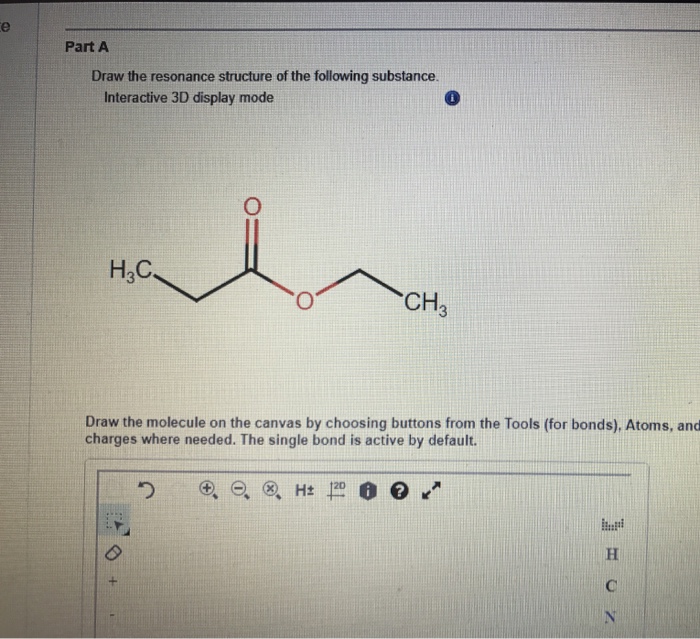
Solved Draw The Resonance Structure Of The Following Chegg Com

Chemistry Net Carbocation Rearrangements Chemistry Advanced Organic Chemistry Organic Chemistry Reactions
0 comments
Post a Comment